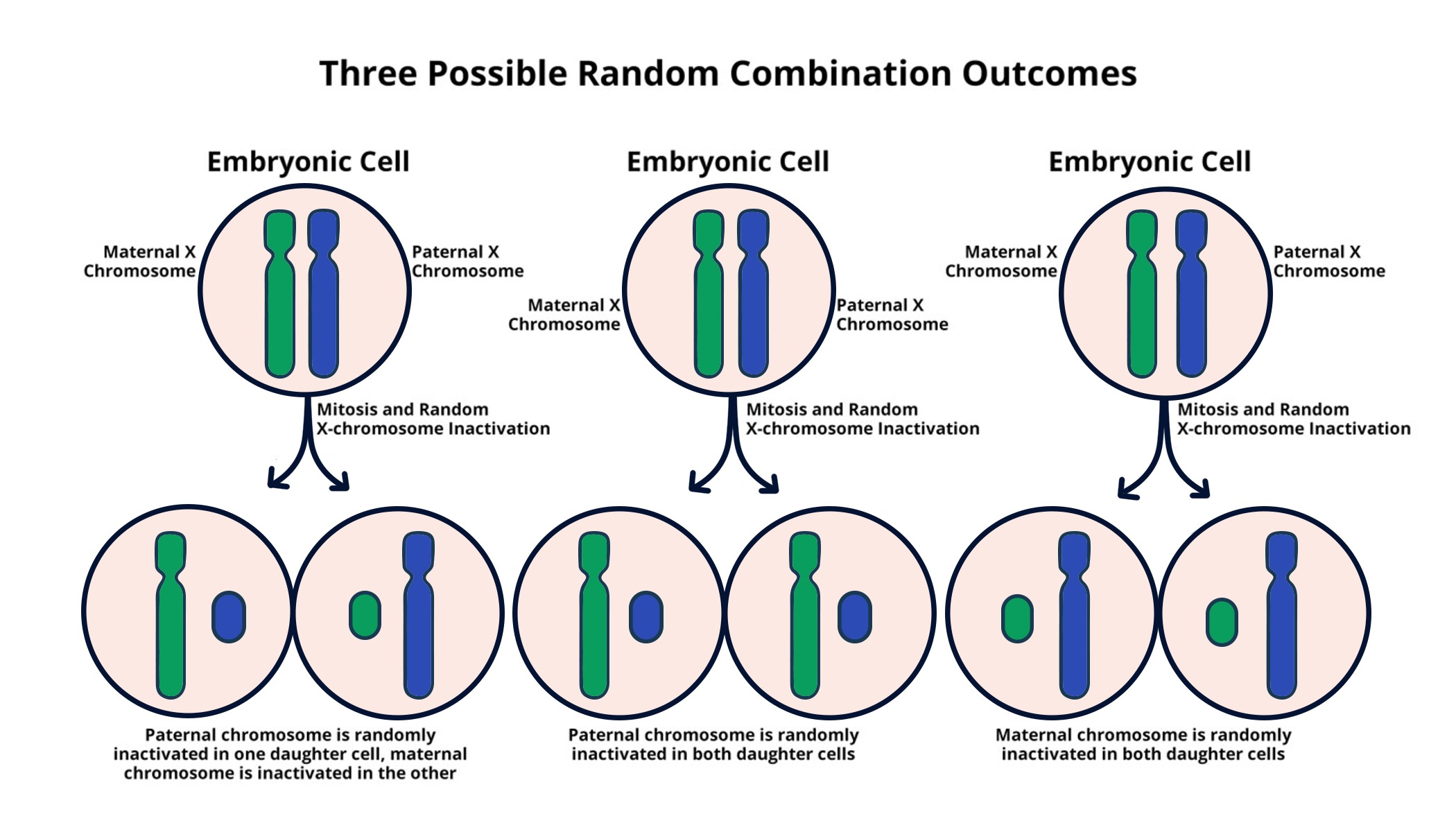
X chromosome inactivation is a fascinating biological process that plays a crucial role in balancing gene expression between females and males. Unlike most chromosomes, which come in pairs regardless of sex, females possess two X chromosomes, but only one is actively utilized. This intricate regulation ensures that females do not have a double dosage of genes encoded on the X chromosome, preventing potential complications. New research spearheaded by Jeannie T. Lee at Harvard Medical School sheds light on the mechanisms of X chromosome inactivation, revealing its implications for genetic disorders such as Fragile X Syndrome and Rett Syndrome. Understanding how this chromosomal breakthrough allows scientists to target inactivated genes paves the way for innovative gene therapy approaches that could pave the road toward effective treatments.
Inactivation of one X chromosome in females, also known as random X inactivation, is a key mechanism for maintaining genetic balance in the cell. This biological strategy ensures that despite having two X chromosomes, a female’s cells behave similarly to those of a male, who has only one. The process hinges on the production of Xist RNA, which alters the chromosomal environment, enabling the silencing of one X chromosome. This research is not only critical for grasping the basics of gene expression but also holds promise for combating genetic conditions linked to mutations on the X chromosome, such as Fragile X Syndrome and Rett Syndrome. By unlocking the secrets of X chromosome inactivation, scientists can potentially utilize these insights for targeted gene therapy interventions.
Understanding X Chromosome Inactivation: The Key to Genetic Therapies
X chromosome inactivation (XCI) is a crucial biological process that ensures dosage compensation between males and females. In females, with two X chromosomes, one X is randomly inactivated during early embryonic development. This process is essential for preventing the over-expression of X-linked genes, which could lead to imbalances that affect cellular function. Scientists like Jeannie Lee have been unraveling how this intricate silencing mechanism operates, particularly the role of Xist RNA, which is vital in orchestrating the inactivation of the X chromosome.
The understanding of X chromosome inactivation not only sheds light on fundamental biology but also opens pathways for innovative gene therapies. By targeting the genes involved in the inactivation process, researchers aim to reactivate beneficial genes that could correct genetic disorders linked to X chromosome mutations, such as Fragile X Syndrome and Rett Syndrome. Advances in this field could revolutionize treatment outcomes, offering hope to those affected by these debilitating conditions.
The Role of Xist RNA in Chromosomal Silencing
Xist RNA is one of the pivotal elements in the process of X chromosome inactivation. It serves as a regulatory RNA that binds to the X chromosome, prompting a cascade of changes that ultimately render that chromosome inactive. As Xist interacts with the surrounding ‘Jell-O’ substance, it alters its mechanical properties, facilitating the inactivation process. This dramatic shift helps other inactivation-related molecules access regions of the X chromosome that would typically remain isolated, thereby establishing the necessary environment for silencing specific genes.
The intricate function of Xist RNA highlights its potential as a target for gene therapy. If scientists can harness this RNA or mimic its function, it could lead to groundbreaking strategies to treat X-linked disorders. The ongoing research by Lee and her associates suggests that leveraging the power of Xist could correct the expression of mutated genes in conditions like Fragile X Syndrome. By manipulating the pathways of Xist RNA, the possibility of therapeutic interventions becomes more tangible, offering a beacon of hope for affected individuals.
Chromosomal Breakthroughs: Insights from Recent Studies
Recent advancements in understanding the mechanics of X chromosome inactivation have yielded chromosomal breakthroughs that promise to transform therapeutic approaches for genetic disorders. The gelatinous substance described by Jeannie Lee and her team plays a vital role in the proper function of chromosomes, ensuring that they are not tangled and can effectively undergo the necessary inactivation processes. By discovering the molecular intricacies at play, researchers can pave the way for novel treatments that address the root causes of conditions like Fragile X Syndrome and Rett Syndrome.
These discoveries are propelling forward the field of gene therapy, as scientists explore methods to temporarily unsilence genes within the inactivated X chromosome. With a better understanding of how X chromosome dynamics work at the cellular level, it becomes increasingly plausible to activate the healthy gene counterpart that can replace mutant expressions. Such targeted therapies offer the promise of not just alleviating symptoms but providing long-term solutions for genetic conditions that have long been deemed incurable.
Implications of Gene Therapy for Fragile X and Rett Syndromes
The potential implications of gene therapy for diseases like Fragile X Syndrome and Rett Syndrome are profound. These X-linked disorders stem from mutations that predominantly affect males, but they also impact females who might carry mutations on one X chromosome. With Lee’s research focusing on unlocking the inactivated X chromosome, gene therapy could enable the expression of healthy genes that compensate for their mutated counterparts. This has the potential to significantly improve cognitive and developmental outcomes for affected individuals.
Furthermore, the ongoing optimization of the therapeutic approaches by the Lee lab signifies a step towards clinical trials that could bring these novel therapies into mainstream medicine. The goal to explore safety and efficacy is paramount, as researchers aim to ensure that their gene therapies provide relief without adverse side effects, preserving the functionality of the remaining healthy genes. This foresight into gene interventions could set a precedent for future treatments targeting other genetic disorders beyond those linked to the X chromosome.
Navigating the Molecular Mechanisms of XCI
Understanding the molecular mechanisms that govern X chromosome inactivation (XCI) is crucial for advancing gene therapy. The inactivation process illustrates how cells can selectively silence one X chromosome while maintaining the functionality of the other in females. Jeannie Lee’s investigations into the underlying dynamics of Xist RNA and the gelatinous material surrounding chromosomes have elucidated the strategies cells employ to prevent gene dosage imbalance, which is vital in diverse biological contexts.
By dissecting these molecular pathways, researchers are not only contributing to a fundamental understanding of genetics but are also laying the groundwork for therapeutic innovations. As more is learned about the intricacies of X chromosome behavior, potential interventions can be designed to target specific genes, providing new hope for treating conditions stemming from X-linked mutations. Innovations in this area inspire a future where targeted gene therapies can be personalized for individuals suffering from genetic disorders.
Future Research Directions in Gene Therapy
The promising results from recent studies underscore the urgency of continued research into gene therapy, particularly as it relates to X chromosome inactivation. Future research directions will focus on optimizing the methods developed by Lee’s lab to ensure their effectiveness across a diverse population. Understanding the variation in response to these therapies will be essential for tailoring interventions that can accommodate different genetic backgrounds and severity of symptoms.
Additionally, researchers are likely to explore the broader implications of their findings beyond Fragile X and Rett Syndromes. The mechanisms of XCI could provide insights into a range of other neurodevelopmental disorders and perhaps even other genetic conditions with chromosomal components. As the field progresses, collaborative efforts will be pivotal in translating laboratory findings into clinical applications that can genuinely impact patient care and improve genetic literacy.
Safety Studies and Clinical Trials: The Next Steps
Before novel therapies targeting X chromosome inactivation can become widely available, comprehensive safety studies are imperative. Potential side effects must be systematically evaluated to ensure any gene therapy approach does not inadvertently disrupt other cellular functions. As Lee and her team prepare to move toward clinical trials, establishing rigorous testing protocols will be crucial to validate the effectiveness and safety of their treatments for Fragile X and Rett Syndromes.
The outcomes of these safety studies will not only inform the next phases of clinical research but will also help in understanding how gene therapies might be adapted for broader use in other genetic disorders. As findings from these trials emerge, they could establish benchmarks for gene therapy efficacy, guiding the development of treatments that utilize similar principles of X chromosome manipulation to address various heritable conditions.
Impacts of X Inactivation Understanding on Genetic Disorders
The research surrounding X chromosome inactivation has transformative potential in understanding and treating genetic disorders. With insights gained about how this process occurs, particularly through the influential role of Xist RNA, clinicians are beginning to fathom how to reactivate inactivated genes effectively. This knowledge base is essential for developing therapies that can correct gene expression in disorders like Fragile X Syndrome and Rett Syndrome, which are often causative due to mutations on the X chromosome.
A deeper understanding of gene silencing and expression dynamics not only aids in targeting specific genetic mutations but also enhances our grasp of how multifactorial genetic disorders arise. The strategies devised to manipulate XCI provide a framework for approaching other genetic conditions, potentially unveiling cures that confront these challenging diseases at their roots.
The Path Forward: From Research to Therapeutics
The transition from groundbreaking research to actionable therapeutics demands a multi-faceted strategy. With Jeannie Lee’s lab illuminating the mechanisms of X chromosome inactivation, the path forward involves rigorous testing, validation, and collaboration with clinical experts. Moving from theoretical understanding to practical application is essential for transforming innovative therapies into effective treatments that patients can access.
The next few years will be critical as research advances move closer to tangible therapeutic options for individuals suffering from genetic disorders linked to X chromosome mutations. Stakeholders across academia, healthcare, and industry must work together to ensure that these discoveries translate into real-world benefits, bringing hope to families affected by Fragile X Syndrome, Rett Syndrome, and beyond.
Frequently Asked Questions
What is X chromosome inactivation and why is it important?
X chromosome inactivation is a biological mechanism that occurs in female mammals, where one of the two X chromosomes is rendered inactive to prevent the overexpression of X-linked genes. This process is crucial because it ensures that females, who have two X chromosomes, do not produce double the amount of gene products compared to males, who have only one X chromosome. Understanding this mechanism is essential for addressing X-linked genetic disorders like Fragile X Syndrome and Rett Syndrome.
How does Xist RNA play a role in X chromosome inactivation?
Xist RNA is a pivotal molecule in the X chromosome inactivation process. It is produced by a gene located on the X chromosome and is responsible for altering the surrounding chromosomal material, often referred to as ‘Jell-O.’ By changing the properties of this substance, Xist enables the inactivation of the X chromosome, effectively silencing it. This process is critical for balancing gene expression between sexes.
Can X chromosome inactivation be targeted for gene therapy in Fragile X Syndrome?
Yes, targeting X chromosome inactivation holds potential for gene therapy in disorders like Fragile X Syndrome. Since this condition is linked to mutations on the X chromosome, methods developed by researchers, such as Jeannie T. Lee’s lab, aim to reactivate the silenced, healthy copies of genes that could provide relief from symptoms. This approach may bring new hope for effective treatments by leveraging the natural inactivation process.
What recent advances have been made in understanding X chromosome inactivation related to Rett Syndrome?
Recent advances in understanding X chromosome inactivation, particularly in the context of Rett Syndrome, have involved uncovering the role of Xist RNA and the biophysical properties of chromosomal material surrounding the X chromosome. Discoveries have indicated that manipulating this inactivation could allow for the expression of healthy genes within the inactivated X chromosome, potentially offering therapeutic strategies for conditions like Rett Syndrome.
How may breaking the silencing of inactivated X chromosomes provide insights into gene therapy?
Breaking the silencing of inactivated X chromosomes could reveal how to utilize healthy genes that are normally inaccessible due to X chromosome inactivation. Research is ongoing to determine whether restoring gene function without affecting healthy genes can lead to effective treatments for X-linked disorders like Fragile X Syndrome and Rett Syndrome. This approach could optimize gene therapy to target specific mutations while preserving normal gene activity.
What challenges remain in the field of X chromosome inactivation research?
Despite significant progress, challenges remain in fully understanding X chromosome inactivation. For example, it is still unclear why inactivated X chromosomes can be reactivated without disrupting the normal function of other genes. Continued research is necessary to explore the mechanisms behind these phenomena and to develop safe and effective therapies that leverage X chromosome inactivation for treating X-linked disorders.
| Key Point | Details |
|---|---|
| X Chromosome Inactivation | Females have two X chromosomes, but only one is active in each cell. |
| Role of Xist RNA | Xist RNA is essential for silencing one of the X chromosomes. |
| Jell-O-Like Substance | A gelatinous substance surrounding chromosomes helps prevent tangling and facilitates X chromosome inactivation. |
| Therapeutic Potential | Methods to unsilence inactivated X chromosomes could lead to treatments for Fragile X and Rett syndromes. |
| Current Research | Ongoing studies focus on optimizing treatments and advancing to clinical trials. |
| Future Insights | Understanding of X-inactivation may help avoid side effects and restore gene function effectively. |
Summary
X chromosome inactivation is a crucial biological process that enables female cells to silence one of their two X chromosomes, ensuring gene dosage balance with males. Recent breakthroughs by Jeannie T. Lee’s team have uncovered the intricate mechanics of this process, revealing the role of Xist RNA and a gelatinous substance that aids in chromosomal organization. As researchers harness this understanding, the potential for developing therapies for X-linked disorders like Fragile X and Rett syndromes is becoming increasingly tangible, promising hope for effective treatments that may minimize side effects.